Terpenes are aromatic compounds found in many plants, including cannabis48,49. They are bioactive, which means they have observable effects on the body.
“These aromatic compounds create the characteristic and scent of many plants, such as cannabis, pine, and lavender, as well as fresh orange peel. The fragrance of most plants is due to a combination of terpenes. In nature, these terpenes protect animals from grazing or infectious germs.”48
Terpenes are often isolated and used to add scent to products such as soap, shampoo, lotion, perfume — and even food (natural beverage flavoring, etc…). They play a key role in the aromatherapy industry, just as they do in the cannabis industry.
Current research on the entourage effect supports the theory that terpenes may alter how phytocannabinoids are processed and used throughout the body. Similar research has also demonstrated that terpenes offer unique benefits that cause each strain of cannabis to have different medicinal properties48.
While there is an array of different terpenes found throughout nature, only a select few have been identified and researched. Many of the terpenes are in critical need of more research to better understand their role in altering/enhancing the effects of cannabis.
It is also worth noting the terpene profile and concentrations of cannabis will vary depending on how it was processed — just as the cannabinoid ratios will vary depending on how the cannabis was processed, as well48,1.
Additionally, some cannabis products contain added terpenes98,99. These are either terpenes that have been “added back” to make up for any that were lost during the extraction process, or terpenes that have been added to enhance the flavor or therapeutic effects of the products.
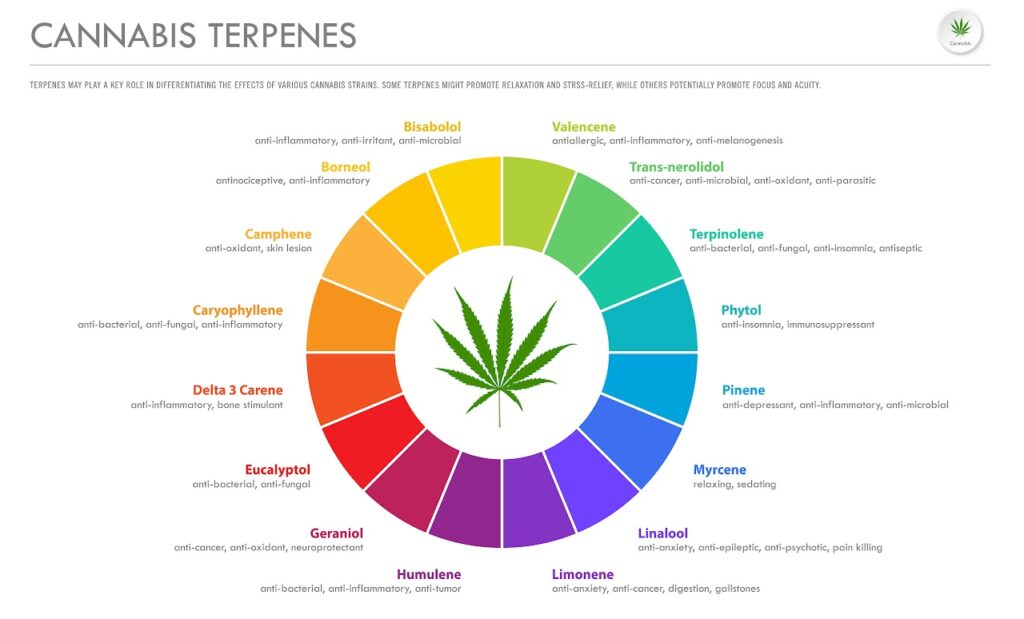
Common terpenes found in cannabis include50:
- Alpha-Bisabolol (α-Bisabolol)
- Beta-Bisabolol (β-Bisabolol)
- Borneol
- Beta-Caryophyllene (β-Caryophyllene)
- Delta-3-Carene (∆-3-Carene)
- Eucalyptol
- Geraniol
- Gamma-Linolenic Acid (γ-Linolenic Acid)
- Humulene (α-Humulene)
- Limonene
- Linalool
- Myrcene
- Trans-nerolidol
- Alpha-Pinene (α-Pinene)
- Beta-Pinene (β-Pinene)
- Ocimene
- Alpha-Terpinene (α-Terpinene)
- Beta-Terpinene (β-Terpinene)
- Gamma-Terpinene (γ-Terpinene)
- Terpineol
- Valencene
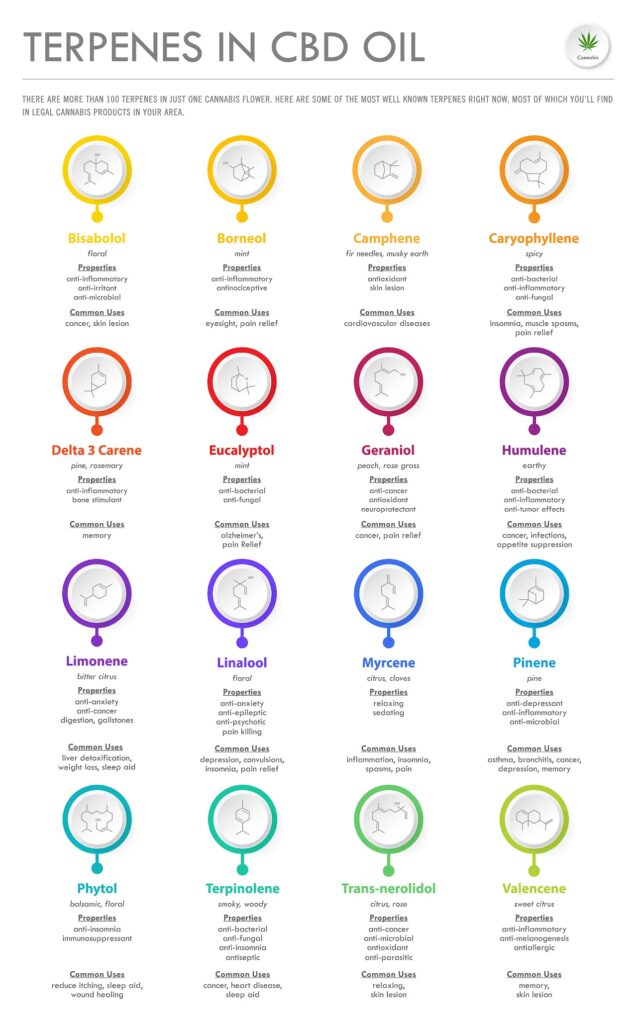
Alpha-Bisabolol (α-Bisabolol)
Found in fats and oils (namely German chamomile oil), α-Bisabolol has a “weak, tangy, fresh and clean, citrus, floral, sweet aroma with a peppery note”34,50.
Benefits associated with α-Bisabolol34:
- Analgesic
- Anti-aging
- Antibacterial
- Anticancer
- Anti-inflammatory
- Ant-irritant
- Antimicrobial
Beta-Bisabolol (β-Bisabolol)
Found in fats and oils (namely cotton oil), β-Bisabolol has a “medium strength citrus, floral, tangy, lemon, fresh, sweet, herbaceous aroma”34. This terpene displays anti-inflammatory, antimicrobial, antifungal, and antimutagenic properties.
Benefits associated with β-Bisabolol34:
- Antifungal
- Antimicrobial
- Antimutagenic (preventing DNA mutation)
Borneol
“Found in several species of Heterotheca, Artemisia, Callicarpa, Dipterocarpaceae, Blumea balsamifera and Kaempferia galanga (all plants, trees, or shrubs),”50 Borneol is a terpene widely (and historically) used throughout Chinese medicine34.
“In Chinese medicine, herbs containing borneol are recommended for overfatigue and stress. Borneol is considered a ‘calming sedative.’ It is directed for fatigue, recovery from illness, and stress. It is found in small quantities in many essential oils”34.
Benefits associated with Borneol34,51:
- Anxiolytic (anti-anxiety)
- Anticoagulant (blood thinners; reduces blood clotting52)
- Sedative and sleep-promoting
Beta-Caryophyllene (β-Caryophyllene)
Found in aromatic oils (namely rosemary and clove oil) and plants including hops, cloves, black pepper, oregano, cinnamon, and basil, Beta-Caryophyllene (β-Caryophyllene) 50,48.
“β-Caryophyllene has a sweet, woody, and dry clove odor and tastes pepper-spicy with camphor and astringent citrus backgrounds.”34 This terpene is widely used throughout the tobacco industry to enhance the flavor of tobacco products, as well.
Benefits associated with β-Caryophyllene34,48,68:
- Topical analgesic (effective for reducing pain via lotions and salves)
- Anticancer
- Antidepressant
- Anxiolytic (anti-anxiety)
- Anti-inflammatory
- Alcohol-craving reduction
Delta-3-Carene (∆-3-Carene)
Found in rosemary, as well as pine and cypress resin, Delta-3-Carene (∆-3-Carene) is used to dry excess bodily fluids in aromatherapy34. It is thought these same properties are responsible for causing dry mouth or dry eyes, which are very common side effects described by cannabis users.
Benefits associated with ∆-3-Carene34,53,54:
- Decreased production of bodily fluids (tears, mucus, sweat, menstrual flow, etc…)
- Anti-inflammatory
- Improved memory and cognition
- Accelerated bone repair and growth
Eucalyptol
As its name implies, eucalyptol has the “camphor-minty odor of eucalyptus”34. Eucalyptol is most commonly found in the eucalyptus tree, as well as rosemary, sage, sweet basil, bay leaves, tea trees, and cardamom50. This terpene is also the main ingredient in eucalyptus oil.
Benefits associated with Eucalyptol34,55:
- Pro-circulatory (increases circulation)
- Topical anti-inflammatory
- Antioxidant
Geraniol
Found in a variety of fruits, vegetables, and essential oils56, geraniol has a “medium strength, floral, sweet, rosey, fruity odor with citrus to citronella-like odor nuances. Its flavor is floral, rosy, waxy, and perfumey with a peach-like nuance.”34
Benefits associated with Geraniol34,56,76:
- Anticancer, anti-tumor
- Anti-fungal
- Anti-inflammatory
- Antimicrobial
- Antioxidant
- Antiviral
- Neuroprotective
“Geraniol has also been shown to sensitize tumor cells to commonly used chemotherapies and represents a promising cancer chemopreventive agent. Due to its anticancer effects, geraniol has been found to be effective against a broad range of cancers including breast, lung, colon, prostate, pancreatic, skin, liver, kidney and oral cancers.”56
Gamma-Linolenic Acid (γ-Linolenic Acid, GLA)
“This omega 6 fatty acid is primarily found in the seed oils of evening primrose, black currant, borage, and fungal oils. This substance can be found in Spirulina (blue-green algae) and hempseed.”50 It has a “slight vegetable oil aroma.”34
Benefits associated with γ-Linolenic Acid34,50,57:
- “Slightly anti-inflammatory”34
- Effective topical treatment for systemic skin conditions, such as eczema, psoriasis, and sclerosis
- Antiproliferative58 (inhibits cell growth, particularly of cells around malignant growths and tumors59)
“Gamma linolenic acid (GLA) is used for conditions that affect the skin including systemic sclerosis, psoriasis, and eczema. It is also used for rheumatoid arthritis (RA), polyps in the mouth, high cholesterol and other blood fats, heart disease, metabolic syndrome (Syndrome-X), diabetic nerve pain, attention deficit-hyperactivity disorder (ADHD), depression, depression after childbirth, chronic fatigue syndrome (CFS), and hay fever (allergic rhinitis). Some people use it to prevent cancer and to help breast cancer patients respond faster to treatment with the drug tamoxifen.”60
Humulene (α-Humulene)
Formerly known as “alpha-caryophyllene (α-caryophyllene),” humulene (α-humulene) has a “bitter, medium woody, and hoppy” aroma34. It is commonly found in the hops of Humulus lupulus, sage, and ginseng61.
Benefits associated with Humulene (α-Humulene)34
“Τhere’s more to humulene than its herbaceous charm. This terpene is found in a wide variety of plants and has been used for centuries in holistic Eastern medicinal practices.”62
Limonene
Found in citrus peels and the rinds of other tropical fruits, limonene is the “second, third or fourth most prevalent terpene in cannabis resins. It is a precursor to the synthesis of other cannabinoids.”34
Benefits associated with Limonene34,50,63:
- Antibacterial
- Anticancer, anti-tumor, chemo-preventive (chemo-preventive means this terpene prevents cancer from developing64)
- Anti-depressant, mood-boosting, energizing
- Antifungal
- Affects cell permeability, allowing THC to reach brain cells and increase the absorption of other terpenes
- Increases blood pressure
“Limonene has been used clinically to dissolve gallstones, improve mood, and relieve heartburn and gastrointestinal reflux. Limonene has been shown to destroy breast-cancer cells in lab experiments, and its powerful antimicrobial action can kill pathogenic bacteria.”34
Linalool
Found in lavender, jasmine, rosewood, basil, and thyme50, linalool has a “floral scent reminiscent of spring flowers such as Lily of the Valley, but with spicy overtones.”34
Benefits associated with Linalool34:
- Anxiolytic (anti-anxiety, reduces stress and anxiety)
- Anticonvulsant
- Antimicrobial65
- Calming
- Effective topical treatment for acne and burns, reduces scarring
- Sedative
Researchers are currently investigating linalool for its potential role in treating a variety of different cancers34.
Myrcene (β-Myrcene, Beta-Myrcene)
Commonly found in fragrant plants, including basil, bay laurel leaves, hops, herbs, lemongrass mangoes, and thyme50, myrcene (β-Myrcene, Beta-Myrcene) has an earthy aroma with “hops, tropical, mango, and minty nuances”34.
β-Myrcene is the most prevalent terpene in throughout the majority of currently available cannabis strains34. It is also widely used throughout the perfume industry.
Benefits associated with β-Myrcene34,50:
- Analgesic
- Anticarcinogenic
- Antidepressant
- Anti-inflammatory
- Antimicrobial
- Antioxidant
- Antiseptic (antiseptic means β-Myrcene stops or slows the growth of microorganisms, reducing the risk of disease or infection67)
- Affects cell permeability, allowing THC to reach brain cells and increase the absorption of other terpenes
- Muscle relaxant
- Prevents liver cancer
- Sedative
“Myrcene is a synergist of THC: A combination of the two molecules creates a stronger experience than THC alone… This terpene contributes strongly to the infamous ‘couch-lock’ experience.”34
Trans-nerolidol
Found in ginger, jasmine, tea tree oil, and yellow grass50, Trans-nerolidol has a “mild, delicate odor that is floral, apple, rose, green, and citrus-like with woody, waxy nuances. Its flavor has been described as green, floral, and woody with fruity-citrus and melon nuances.”34
Benefits associated with Trans-nerolidol34,68:
- Antifungal
- Antileishmaniasis (Leishmaniasis is a parasitic disease found in parts of the tropics, subtropics, and southern Europe69. The infection is caused by Leishmania parasites, which are spread through bites from infected sandflies)
- Antimicrobial
- Antiparasitic
- Sedative
Alpha-Pinene (α-Pinene) & Beta-Pinene (β-Pinene)
Commonly found in pine trees, eucalyptus, rosemary, sage, and turpentine, alpha-pinene (α-pinene) has an indisputable pine tree scent34. Similarly, β-pinene (beta-pinene) has a “woody-green pine-like smell,” and it is one of the most abundant compounds released by forest trees.
α-pinene and β-pinene are each one of the two isomers of pinene and share similar properties. Isomers are two molecules with a similar formula but different structure70. Thus, α-pinene and β-pinene have the same number of atoms for each element, but their atomic arrangement is different.
Benefits associated with Alpha-Pinene (α-Pinene) and Beta-Pinene (β-Pinene)34,71:
- Analgesic
- Antibiotic resistance modulation (useful for treating antibiotic resistant bacteria, such as MRSA)
- Anticoagulant (blood thinners; reduces blood clotting52)
- Anti-inflammatory
- Anti-malarial
- Antimicrobial
- Antioxidant
- Antileishmaniasis (Leishmaniasis is a parasitic disease found in parts of the tropics, subtropics, and southern Europe69. The infection is caused by Leishmania parasites, which are spread through bites from infected sandflies)
- Bronchodilator (Useful for treating asthma50)
- Promotes alertness, focus, and memory retention
“Largely due to the presence of pinene, rosemary and sage are both considered ‘memory plants.’ Concoctions made from their leaves have been used for thousands of years in traditional medicine to retain and restore memory… α-Pinene has inhibited acetylcholinesterase suggesting utility in the clinical treatment of Alzheimer’s disease.”34
Ocimene
Found in basil, bergamot, kumquats, lavender, mangoes, orchids, and peppers, ocimene has a “sweet, floral, and herbaceous aromatic profile.”72
Benefits associated with Ocimene34,72:
- Anti-inflammatory
- Antioxidant
“Further research indicates that ocimene may help treat symptoms of diabetes by inhibiting the proliferation of key enzymes connected to type 2 diabetes and hypertension.”
Hypertension is high blood pressure (whereas hypotension is low-blood pressure)73.
Alpha-Terpinene (α-Terpinene), Beta-Terpinene (β-Terpinene), & Gamma-Terpinene (γ-Terpinene)
Found in allspice, cardamom, citrus, eucalyptus, juniper, marjoram, and tea tree oil50, alpha-terpinene (α-terpinene) has a “refreshing, lemony-citrus aroma.”34 It is one of three isomers of terpinene, with β-terpinene and γ-terpinene serving as the other two isomers74.
Considering that α-terpinene, β-terpinene, and γ-terpinene are isomers, it is likely that they share similar benefits.
Benefits associated with Alpha-Terpinene (α-Terpinene), Beta-Terpinene (β-Terpinene), and Gamma-Terpinene (γ-Terpinene)34,74
- Anti-inflammatory
- Antimicrobial
- Antioxidant
- Antiproliferative
Tea tree oil — which contains terpinene — has historically been used as an antibacterial, antifungal, and antiseptic agent for centuries. Therefore, these properties may be due to terpinene (or another terpinene)34. However, further testing on isolated terpinene to evaluate its standalone antibacterial, antifungal, and antiseptic properties is needed.
Terpineol
Found in eucalyptus sap, lilacs, lime blossoms, pine trees, and other plants50, terpineol has a floral, fruity scent known for its ability to “relax consumers.”34,76 Terpineol is most commonly found in strains that are high in pinene, which tends to overpower the scent of terpineol76.
Benefits associated with Terpineol34,76:
- Antibiotic
- Anti-inflammatory
- Anti-malarial
- Antioxidant
- Anti-tumor
- Anxiolytic (anti-anxiety, stress relieving)
- Sedative (Terpineol may contribute to the ‘couch-lock’ effect associated with some strains of cannabis)
Terpinolene
Found in apples, cumin, lilacs, nutmeg, and tea tree, terpinolene has a fresh, woody, sweet, and piney aroma with a hint of citrus34,77. “Its flavor is sweet, woody, terpy, lemon and lime-like with a slight herbal and floral nuance.”34
“Terpinolene is a lurker. It’s found in plenty of cannabis strains, but it’s usually present only in small amounts. It may, in fact, be the least-common common terpene — often among a strain’s cast of characters, but rarely in a leading role.”77
Benefits associated with Terpinolene77:
- Antibacterial
- Antifungal
- Repels pests including mosquitoes and weevils
Current research is investigating terpinolene’s potential to reduce the risk of heart disease as well as terpinolene’s potential to inhibit cancer cell growth.
“[Terpinolene’s] use in fragrance in the USA alone exceeds 50,000 lbs/year. Terpinolene is used in soap, detergent, creams, lotions, and perfumes.”34
Valencene
Most commonly found in Valencia oranges, Valencene has a sweet, citrusy aroma and flavor76.
Benefits associated with Valencene76,78:
- Anti-allergic (preventing allergic responses79)
- Anti-inflammatory
- Bronchodilator
- Insectifungal (killing insects and fungi)
- May protect skin from sun-related/UV damage80
Research on Valencene is still in its infancy. It’s likely there’s still plenty left to uncover about this terpene.
The Bioavailability of Terpenes
How terpenes exert their therapeutic effects depends on the route of administration (i.e. were they inhaled through a vapor product? Consumed in an edible? Applied topically in a lotion?)98.
The term “bioavailability” refers to the rate and fraction of the initial dose of a substance that reaches its intended biological target100. In this case, the “bioavailability” of terpenes refers to how much of the terpene is actually absorbed and utilized by the body.
Below, we have broken down the mechanisms based on how terpenes may be consumed.
Inhalation
When vaping or smoking terpenes, they’re quickly absorbed by your lungs and enter directly into your bloodstream98. The bioavailability range of the terpenes α-pinene (alpha-pinene), camphor, and menthol is 54 to 76 percent. According to the National Cannabis Industry Association, this is relatively high when compared to their oral bioavailability.
Ingestion
Various factors affect the bioavailability of terpenes, such as your pH balance and the actions of your digestive enzymes98. According to the NCIA, terpenes are absorbed by the gastrointestinal tract and become bioavailable within 30 minutes of first consuming them. Terpenes reach their peak bioavailability within two to four hours of ingestion.
Topical Application
Terpenes have many dermatological benefits, such as their anti-inflammatory, anti-acne, and wound-healing properties98. According to the NCIA, terpenes “easily penetrate the skin and enhance transdermal delivery.” Furthermore, terpenes may potentially aid in the transdermal delivery of cannabinoids.
The bioavailability of terpenes delivered transdermally ranges from three to 12 percent, depending on the type of terpene it is and the topical formulation. Terpenes reach their maximum plasma levels within 10 minutes of topical application.
Safety Guidelines & Limits for Added Terpenes
The recommended safety guidelines and limits for added terpenes vary depending on the route of administration, as well98.
Inhalation Safety Guidelines & Terpene Limits
As a rule of thumb, the NCIA recommends the concentration of terpenes shouldn’t exceed more than 10 percent in the final product98. For reference, the natural ratios of terpenes in cannabis range from one to five percent.
When vaporizing terpenes, it’s important it is done in a device with an adjustable temperature setting. Using a lower temperature to vaporize terpenes prevents “unnecessary heat-derived toxin production” from exposure to higher temperatures.
Furthermore, terpenes are natural compounds that should be used within the intended expiration date on the product’s packaging. Laboratory testing is essential to ensure the quality, safety, and stability of the product.
The terpene limits suggested by the NCIA are based on the ANEC Position Paper on e-cigarettes and e-liquids. It’s important to note the average e-cigarette consumer takes 500 “puffs” per day, while the average cannabis consumer takes nine puffs per day. Thus, the potential safe limits could be higher than outlined below, since cannabis consumers tend to use their cartridges significantly fewer times per day than the average e-cigarette consumer.
- α-Terpineol (Alpha-Terpineol) limit per e-liquid: 1.1%
- β-Pinene (Beta-Pinene) limit per e-liquid: 0.7%
- Carvone limit per e-liquid: 0.14%
- Geranyl Acetate limit per e-liquid: 7.4%
- Linalool limit per e-liquid: 0.34%
- Menthol limit per e-liquid: 7.8%
Ingestion Safety Guidelines & Terpene Limits
Terpenes are common flavor additives in food products98. However, the exact dosage for therapeutic benefits in edible products is not yet fully understood and warrants further research. It’s also vital to ensure terpenes that are added to edible cannabis products are food-grade terpenes backed by a certificate of analysis to verify they’re safe to ingest.
The NCIA bases its safe terpene limits on the Food and Extract Manufacturers of the United States’ program. FEMA’s program relies on the GRAS (“generally recognized as safe”101) concept to determine the safety of flavoring substances. The program has developed different safety recommendations for added terpenes depending on the type of consumable product and measures the terpenes added by their parts per million (ppm) in the product.
Parts per million can also be thought of as how many milligrams per liter (mg/L), or as a percentage where 10,000 mg = 1%.
- Non-Alcoholic Beverages:
- Lime Terpenes (Average Maximum PPM): 750
- Orange Terpenes (Average Maximum PPM): 1,550
- Grapefruit Terpenes (Average Maximum PPM): 500
- Limonene (Average Maximum PPM): 31
- Myrcene (Average Maximum PPM): 4.4
- Linalool (Average Maximum PPM): 7
- Alcoholic Beverages:
- Lime Terpenes (Average Maximum PPM): 1,000
- Orange Terpenes (Average Maximum PPM): 1,000
- Grapefruit Terpenes (Average Maximum PPM): 1,000
- Limonene (Average Maximum PPM): N/A
- Myrcene (Average Maximum PPM): N/A
- Linalool (Average Maximum PPM): 50
- Chewing Gum:
- Lime Terpenes (Average Maximum PPM): 20,000
- Orange Terpenes (Average Maximum PPM): 20,000
- Grapefruit Terpenes (Average Maximum PPM): 20,000
- Limonene (Average Maximum PPM): 2,300
- Myrcene (Average Maximum PPM): N/A
- Linalool (Average Maximum PPM): 200
- Hard Candy:
- Lime Terpenes (Average Maximum PPM): 5,000
- Orange Terpenes (Average Maximum PPM): 5,000
- Grapefruit Terpenes (Average Maximum PPM): 5,000
- Limonene (Average Maximum PPM): 49
- Myrcene (Average Maximum PPM): 13
- Linalool (Average Maximum PPM): 400
- Soft Candy:
- Lime Terpenes (Average Maximum PPM): 5,000
- Orange Terpenes (Average Maximum PPM): 5,000
- Grapefruit Terpenes (Average Maximum PPM): 5,000
- Limonene (Average Maximum PPM): N/A
- Myrcene (Average Maximum PPM): N/A
- Linalool (Average Maximum PPM): 10
The NCIA also cites safety recommendations from the European Chemical Agency, which bases the safety of ingested terpenes on the “Daily No Effect Level” (DNEL). The DNEL is calculated based on the milligrams of terpenes consumed in proportion to an individual’s body weight in kilograms.
- α-Terpineol (Alpha-Terpineol) DNEL: “No hazard identified”
- β-Pinene (Beta-Pinene) DNEL: 0.3mg/kg bw
- Carvone DNEL: 69.4μg/kg bw
- Geranyl Acetate DNEL: 8.9mg/kg bw
- Linalool DNEL: 0.2mg/kg bw
- Menthol DNEL: 4.7 mg/kg bw
Topical Safety Guidelines & Terpene Limits
Although terpenes contain many known skin benefits, some are also dermal irritants98. The intensity of the irritation depends on how concentrated the terpene is, so these allergenic terpenes must be diluted. Oxidization also increases the risk of causing a skin reaction, as the oxidized forms of terpenes are more reactive.
Below is the list of allergenic terpenes highlighted by the NCIA. Since these terpenes are considered common allergens, the packaging should clearly state the presence of these terpenes if it’s potentially higher than 0.01% in “rinse-off” products (shower & bath gels) or 0.0001% in “leave-on” products (massage oils, body oils, and creams).
- Citral
- Citronellol
- Eugenol
- Farnesol
- Geraniol
- Isoeugenol
- D-Limonene
- Linalool
The NCIA’s terpene concentration limits for topical products are based on the recommendations made by the International Fragrance Association. The limits vary depending on the type of topical product it is and which terpene it is.
- α-Bisabolol (Alpha-Bisabolol):
- Lip Products: 0.42%
- Body Lotions, Creams, & Oils: 2.40%
- Hand Sanitizers & Hand Creams: 0.60%
- Body Washes: 4.60%
- Citral:
- Lip Products: 0.11%
- Body Lotions, Creams, & Oils: 0.60%
- Hand Sanitizers & Hand Creams: 0.15%
- Body Washes: 1.2%
- Citronellol:
- Lip Products: 2.20%
- Body Lotions, Creams, & Oils: 12.00%
- Hand Sanitizers & Hand Creams: 3.20%
- Body Washes: 24.0%
- Eugenol:
- Lip Products: 0.45%
- Body Lotions, Creams, & Oils: 2.50%
- Hand Sanitizers & Hand Creams: 0.64%
- Body Washes: 4.90%
- Farnesol:
- Lip Products: 0.21%
- Body Lotions, Creams, & Oils: 1.20%
- Hand Sanitizers & Hand Creams: 0.29%
- Body Washes: 2.30%
- Geraniol:
- Lip Products: 0.85%
- Bod Lotions, Creams, & Oils: 4.70%
- Hand Sanitizers & Hand Creams: 1.20%
- Body Washes: 9.20%
To verify the safety of terpene-infused topical products, the NCIA recommends creating sample products with an infusion of 0.5% to 5% terpenes in petroleum jelly. Patch testing these formulations can help identify potential skin reactions so the formula may be adjusted.
Key Takeaways
Terpenes are aromatic compounds found in many plants, including cannabis48,49. They are bioactive, which means they have observable effects on the body.
Current research on the entourage effect supports the theory that terpenes may alter how phytocannabinoids are processed and used throughout the body. Similar research has also demonstrated that terpenes offer unique benefits that cause each strain of cannabis to have different medicinal properties48.
While there is an array of different terpenes found throughout nature, only a select few have been identified and researched. Many of the terpenes are in critical need of more research to better understand their role in altering/enhancing the effects of cannabis.
It is also worth noting the terpene profile and concentrations of cannabis will vary depending on how it was processed — just as the cannabinoid ratios will vary depending on how the cannabis was processed, as well48,1. Some cannabis products contain added terpenes98,99. These are either terpenes that have been “added back” to make up for any that were lost during the extraction process, or terpenes that have been added to enhance the flavor or therapeutic effects of the products. The NCIA has various safety guidelines and recommended limits for added terpenes in inhalable, ingestible, and topical cannabis products.
Just like cannabinoids, terpenes possess a variety of therapeutic properties. Some terpenes are anti-inflammatory, analgesic, anti-aging, antibacterial, anticancer, antidepressant, antifungal, anxiolytic, and sleep-promoting, to name a few of their medicinal benefits.
Similarly, the bioavailability of terpenes varies depending on how the product is administered. Inhaled terpenes have the highest range of bioavailability, which is between 54 and 76 percent.
References
- Spindle, T. R., Bonn-Miller, M. O., & Vandrey, R. (2019). Changing landscape of cannabis: novel products, formulations, and methods of administration. Current Opinion in Psychology, 30, 98–102. https://doi.org/10.1016/j.copsyc.2019.04.002
- Morales, P., Hurst, D. P., & Reggio, P. H. (2017). Molecular Targets of the Phytocannabinoids: A Complex Picture. Progress in the Chemistry of Organic Natural Products, 103–131. https://doi.org/10.1007/978-3-319-45541-9_4
- WeedMaps. (n.d.). What is a Phytocannabinoid? | Phytocannabinoid Definition by Weedmaps. https://weedmaps.com/learn/dictionary/phytocannabinoid/
- Di Marzo, V., & Piscitelli, F. (2015). The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics, 12(4), 692–698. https://doi.org/10.1007/s13311-015-0374-6
- About time. (n.d.-b). Human CBD Receptor Chart [Infographic]. Adobe Stock. https://stock.adobe.com/images/human-cbd-receptor-chart-endocannabinoid-system-horizontal-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/267901368?asset_id=267901368
- About time. (n.d.-c). Human Endocannabinoid System [Infographic]. Adobe Stock. https://stock.adobe.com/images/human-endocannabinoid-system-endocannabinoid-system-horizontal-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/276860924?prev_url=detail
- Raypole, C., & Carter, A. (2019, May 17). A Simple Guide to the Endocannabinoid System. Healthline. https://www.healthline.com/health/endocannabinoid-system
- Silver, R. J. (2019). The Endocannabinoid System of Animals. Animals, 9(9), 686. https://doi.org/10.3390/ani9090686
- Muller, C., Morales, P., & Reggio, P. H. (2019). Cannabinoid Ligands Targeting TRP Channels. Frontiers in Molecular Neuroscience, 11, 0. https://doi.org/10.3389/fnmol.2018.00487
- Ahimsadasan, N. (2021, January 13). Neuroanatomy, Dorsal Root Ganglion – StatPearls – NCBI Bookshelf. NCBI. https://www.ncbi.nlm.nih.gov/books/NBK532291/
- marina_ua. (n.d.). The nervous system [Graphic]. Adobe Stock. https://stock.adobe.com/images/the-nervous-system/94711659?prev_url=detail
- The Editors of Encyclopaedia Britannica. (2014, January). Osteoclast | cell. Encyclopedia Britannica. https://www.britannica.com/science/osteoclast
- Akopian, A. N., Ruparel, N. B., Jeske, N. A., Patwardhan, A., & Hargreaves, K. M. (2009). Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends in Pharmacological Sciences, 30(2), 79–84. https://doi.org/10.1016/j.tips.2008.10.008
- Atalay, S., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants, 9(1), 21. https://doi.org/10.3390/antiox9010021
- Pellati, F., Borgonetti, V., Brighenti, V., Biagi, M., Benvenuti, S., & Corsi, L. (2018). Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Research International, 2018, 1–15. https://doi.org/10.1155/2018/1691428
- Marrone, M. C., Morabito, A., Giustizieri, M., Chiurchiù, V., Leuti, A., Mattioli, M., Marinelli, S., Riganti, L., Lombardi, M., Murana, E., Totaro, A., Piomelli, D., Ragozzino, D., Oddi, S., Maccarrone, M., Verderio, C., & Marinelli, S. (2017). TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nature Communications, 8(1), 0. https://doi.org/10.1038/ncomms15292
- Centers For Disease Control and Prevention. (2017, August 21). About synthetic cannabinoids. https://www.cdc.gov/nceh/hsb/chemicals/sc/About.html
- Wiley, J., Marusich, J., Huffman, J. W., Balster, R. L., & Thomas, B. (2011). Hijacking of Basic Research: The Case of Synthetic Cannabinoids. RTI Press, 0. https://doi.org/10.3768/rtipress.2011.op.0007.1111
- Peñaloza, M. (2018, July 27). NPR Cookie Consent and Choices. NPR. https://choice.npr.org/index.html?origin=https://www.npr.org/2018/07/27/632261920/d-c-has-had-more-than-300-suspected-k2-overdoses-in-2-weeks
- Maccarrone, M. (2020). Phytocannabinoids and endocannabinoids: different in nature. Rendiconti Lincei. Scienze Fisiche e Naturali, 31(4), 931–938. https://doi.org/10.1007/s12210-020-00957-z
- Rahn, B. (2020, September 29). The entourage effect: How cannabis compounds may be working together. Leafly. https://www.leafly.com/news/cannabis-101/cannabis-entourage-effect-why-thc-and-cbd-only-medicines-arent-g
- Gülck, T., & Møller, B. L. (2020). Phytocannabinoids: Origins and Biosynthesis. Trends in Plant Science, 25(10), 985–1004. https://doi.org/10.1016/j.tplants.2020.05.005
- Beadle, A. (2020, July 27). CBG vs CBD: What Are the Differences? Analytical Cannabis. https://www.analyticalcannabis.com/articles/cbg-vs-cbd-what-are-the-differences-312232
- Bennett, P. (2020a, July 28). THCA and CBD Crystalline: Cannabinoids at Their Purest. Leafly. https://www.leafly.com/news/strains-products/what-are-thca-cbda-crystalline-cannabinoids
- Morano, A., Fanella, M., Albini, M., Cifelli, P., Palma, E., Giallonardo, A. T., & Di Bonaventura, C. (2020). Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatric Disease and Treatment, Volume 16, 381–396. https://doi.org/10.2147/ndt.s203782
- Scaccia, A., & Wilson, D. (2020, August 19). Serotonin: What You Need to Know. Healthline. https://www.healthline.com/health/mental-health/serotonin
- Ameri, A. (1999). The effects of cannabinoids on the brain. Progress in Neurobiology, 58(4), 315–348. https://doi.org/10.1016/s0301-0082(98)00087-2
- Lucas, C. J., Galettis, P., & Schneider, J. (2018, July 12). The pharmacokinetics and the pharmacodynamics of cannabinoids. British Journal of Clinical Pharmacology. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bcp.13710
- National Cancer Institute. (n.d.). NCI Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/delta-8-tetrahydrocannabinol?redirect=true
- Shannon, S. (2019). Cannabidiol in Anxiety and Sleep: A Large Case Series. The Permanente Journal, 23, 0. https://doi.org/10.7812/tpp/18-041
- Grandy, D. (2016). Amphetamines Activate G-Protein Coupled Trace Amine-Associated Receptor 1 (TAAR1). In Neuropathology of drug addictions and substance misuse. (pp. 108–116). Academic Press,. https://doi.org/10.1016/B978-0-12-800212-4.00010-8
- Raypole, C., & Sullivan, D. (2020, March 30). Cannabis Got You Paranoid? How to Deal With It. Healthline. https://www.healthline.com/health/marijuana-paranoia
- Walters, O., & Kerr, M. (2020, October 7). A Straightforward Cannabinoid Chart With Simple Explanations. Sovereignty. https://sovereignty.co/cannabinoid-chart/
- S. (2020, July 15). The Terpenes Of Cannabis, Their Aromas, And Effects. THCFarmer – Cannabis Cultivation Network. https://www.thcfarmer.com/threads/the-terpenes-of-cannabis-their-aromas-and-effects.68222/
- Marijuana Doctors. (2018, November 20). Cannabivarin (CBV) | What Is CBV. https://www.marijuanadoctors.com/resources/cannabinoids/cannabivarin-cbv/
- Pretzsch, C. M., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., Wichers, R., Ajram, L., Ivin, G., Heasman, M., Edden, R. A. E., Williams, S., Murphy, D. G. M., Daly, E., & McAlonan, G. M. (2019). Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): a single dose trial during magnetic resonance spectroscopy. Translational Psychiatry, 9(1), 0. https://doi.org/10.1038/s41398-019-0654-8
- Abioye, A., Ayodele, O., Marinkovic, A., Patidar, R., Akinwekomi, A., & Sanyaolu, A. (2020). Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. Journal of Cannabis Research, 2(1), 0. https://doi.org/10.1186/s42238-020-0016-7
- García, C., Palomo-Garo, C., García-Arencibia, M., Ramos, J. A., Pertwee, R. G., & Fernández-Ruiz, J. (2011). Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. British Journal of Pharmacology, 163(7), 1495–1506. https://doi.org/10.1111/j.1476-5381.2011.01278.x
- Seladi-Schulman, J., & Kay, C. (2019, August 19). In Vivo vs. In Vitro: What Does It All Mean? Healthline. https://www.healthline.com/health/in-vivo-vs-in-vitro
- Coombs, D. W. & The Journal of the American Medical Association. (1983, November 4). antinociception. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/medical/antinociception
- Citti, C., Linciano, P., Russo, F., Luongo, L., Iannotta, M., Maione, S., Laganà, A., Capriotti, A. L., Forni, F., Vandelli, M. A., Gigli, G., & Cannazza, G. (2019). A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Scientific Reports, 9(1), 0. https://doi.org/10.1038/s41598-019-56785-1
- Stone, E. (2020, September 30). Meet THCP and CBDP: Study reveals the identification of two new cannabinoids. Leafly. https://www.leafly.com/news/science-tech/thcp-cbdp-study-reveals-identification-two-new-cannabinoids
- Awakened Tropicals. (2021, March 16). Meet the Raw Cannabinoids. https://www.awakenedtopicals.com/blog/2019/7/10/meet-the-raw-cannabinoids
- About time. (n.d.-e). Phytocannabinoids vs Endocannabinoids [Graphic]. Adobe Stock. https://stock.adobe.com/images/phytocannabinoids-vs-endocannabinoids-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/330635278?prev_url=detail
- About time. (n.d.-g). The Entourage Effect [Graphic]. Adobe Stock. https://stock.adobe.com/images/the-entourage-effect-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/330634809?prev_url=detail
- About time. (n.d.-d). Main Benefits of Marijuana Cannabinoids [Graphic]. Adobe Stock. https://stock.adobe.com/images/main-benefits-of-marijuana-cannabinoids-information-poster-with-benefits-of-marijuana-cannabinoids-and-table-of-natural-cannabinoids/414944091?prev_url=detail
- About time. (n.d.-a). Cannabis Terpenes [Graphic]. Adobe Stock. https://stock.adobe.com/images/cannabis-terpenes-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/316181241?prev_url=detail
- Johnson, J., & Theisen, E. (2020, March 6). What to know about terpenes. Medical News Today. https://www.medicalnewstoday.com/articles/what-are-terpenes
- Sommano, S. R., Chittasupho, C., Ruksiriwanich, W., & Jantrawut, P. (2020). The Cannabis Terpenes. Molecules, 25(24), 5792. https://doi.org/10.3390/molecules25245792
- Feelds. (2021, March). Terpenes — Miroboard [Chart]. Miro. https://miro.com/app/board/o9J_ktrdRac=/
- Johnston, G. A. R., Chebib, M., Duke, R. K., Fernandez, S. P., Hanrahan, J. R., Hinton, T., & Mewett, K. N. (2009). Herbal Products and GABA Receptors. Encyclopedia of Neuroscience, 1095–1101. https://doi.org/10.1016/b978-008045046-9.00868-8
- Shiel, W. C. (2017, March 3). Anticoagulant (Blood Thinner) Medical Definition Written by Doctors. MedicineNet. https://www.medicinenet.com/anticoagulant/definition.htm
- Strain Print. (2020, January 24). Understanding Terpenes: Delta 3 Carene. Strainprint Technologies Inc. https://strainprint.ca/understanding-terpenes-delta-3-carene/
- Robbins, C. (2020, September 8). Delta 3 Carene: The Terpene That Promotes Healthy Bones (& Dry Mouth). Cannabis Aficionado. https://cannabisaficionado.com/delta-3-carene/
- Seol, G. H., & Kim, K. Y. (2016). Eucalyptol and Its Role in Chronic Diseases. Advances in Experimental Medicine and Biology, 389–398. https://doi.org/10.1007/978-3-319-41342-6_18
- National Center for Biotechnology Information. (2021c). Geraniol. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Geraniol
- National Center for Biotechnology Information. (2021b). gamma-Linolenic acid. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5280933
- Fan, Y.-Y., & Chapkin, R. S. (1998). Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. The Journal of Nutrition, 128(9), 1411–1414. https://doi.org/10.1093/jn/128.9.1411
- Dictionary.com. (n.d.). Definition of antiproliferative | Dictionary.com. Www.Dictionary.Com. https://www.dictionary.com/browse/antiproliferative
- RxList. (2019, September 17). Gamma Linolenic Acid: Health Benefits, Uses, Side Effects, Dosage & Interactions. https://www.rxlist.com/gamma_linolenic_acid/supplements.htm
- Hartsel, J. A., Eades, J., Hickory, B., & Makriyannis, A. (2016). Cannabis sativa and Hemp. Nutraceuticals, 735–754. https://doi.org/10.1016/b978-0-12-802147-7.00053-x
- Bennett, P. (2020b, July 28). What is humulene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/science-tech/humulene-terpene
- National Center for Biotechnology Information. (2021d). Limonene. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/22311
- Cancer.Net Editorial Board. (2020, October 21). Chemoprevention. Cancer.Net. https://www.cancer.net/navigating-cancer-care/prevention-and-healthy-living/chemoprevention
- National Center for Biotechnology Information. (2021e). Linalool. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/6549
- About time. (n.d.-f). Terpenes in CBD Oil [Graphic]. Adobe Stock. https://stock.adobe.com/images/terpenes-in-cbd-oil-with-structural-formulas-vertical-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/289977909?prev_url=detail
- Gotter, A., & Murrell, D. (2019, March 7). A Guide to Antiseptics. Healthline. https://www.healthline.com/health/what-is-antiseptic
- Tilray. (2020b, July 28). Cannabis Terpenes: The Benefits of Humulene, Caryophyllene, and Trans-Nerolidol. Leafly. https://www.leafly.com/news/cannabis-101/humulene-caryophyllene-and-trans-nerolidol-what-are-the-benefits
- Centers for Disease Control and Prevention. (2020, May 19). CDC – Leishmaniasis – General Information – Frequently Asked Questions (FAQs). https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html
- BD Editors. (2017, June 13). Isomer. Biology Dictionary. https://biologydictionary.net/isomer/
- Salehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., L.D. Jayaweera, S., A. Dias, D., Sharopov, F., Taheri, Y., Martins, N., Baghalpour, N., C. Cho, W., & Sharifi-Rad, J. (2019). Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules, 9(11), 738. https://doi.org/10.3390/biom9110738
- Bennett, P. (2020c, July 28). What is ocimene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/science-tech/benefits-of-ocimene-terpene
- Mayo Clinic. (2021, January 16). High blood pressure (hypertension) – Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/symptoms-causes/syc-20373410
- National Center for Biotechnology Information. (2021a). alpha-Terpinene. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/7462
- Barnes, S. (2021, February 1). What is Terpinene | Terpinene Definition by. Weedmaps. https://weedmaps.com/learn/dictionary/terpinene
- Tilray. (2020a, July 28). Benefits of Cannabis Terpenes: Terpineol, Valencene, and Geraniol. Leafly. https://www.leafly.com/news/cannabis-101/cannabis-terpenes-terpineol-valencene-geraniol
- Adlin, B. (2020, July 28). What is terpinolene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/strains-products/least-common-terpene-terpinolene-effects
- Lodi, M. (2019, July 24). Terpenes 411: Valencene. MedMen. https://www.medmen.com/blog/guides/terpenes-411-valencene
- Merriam-Webster Dictionary. (1919). anti-allergic. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/dictionary/anti-allergic
- Nam, J. H., Nam, D.-Y., & Lee, D.-U. (2016). Valencene from the Rhizomes of Cyperus rotundus Inhibits Skin Photoaging-Related Ion Channels and UV-Induced Melanogenesis in B16F10 Melanoma Cells. Journal of Natural Products, 79(4), 1091–1096. https://doi.org/10.1021/acs.jnatprod.5b01127
- Rea, K. A., Casaretto, J. A., Al-Abdul-Wahid, M. S., Sukumaran, A., Geddes-McAlister, J., Rothstein, S. J., & Akhtar, T. A. (2019). Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry, 164, 162–171. https://doi.org/10.1016/j.phytochem.2019.05.009
- Gardner, F. (n.d.). The Cannflavins Unique to Cannabis | O’Shaughnessy’s. O’Shaughnessy’s Online. https://beyondthc.com/the-cannflavins-unique-to-cannabis/
- Yang, X., Jiang, Y., Yang, J., He, J., Sun, J., Chen, F., Zhang, M., & Yang, B. (2015). Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends in Food Science & Technology, 44(1), 93–104. https://doi.org/10.1016/j.tifs.2015.03.007
- Merriam-Webster Dictionary. (n.d.). Genus. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/dictionary/genus
- Lipophilicity | Medicinal Chemistry. (n.d.). Omicsonline. https://www.omicsonline.org/lipophilicity-scholarly-open-access-journals.php
- Henderson, R. (2014, November 13). Catatonia and Catalepsy. Patient. https://patient.info/doctor/catatonia-and-catalepsy
- Ricciotti, E., & FitzGerald, G. A. (2011). Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(5), 986–1000. https://doi.org/10.1161/atvbaha.110.207449
- WebMD. (n.d.). Indomethacin Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing – WebMD. https://www.webmd.com/drugs/2/drug-8880-5186/indomethacin-oral/indomethacin-oral/details
- Radwan, M. M., ElSohly, M. A., Slade, D., Ahmed, S. A., Wilson, L., El-Alfy, A. T., Khan, I. A., & Ross, S. A. (2008). Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry, 69(14), 2627–2633. https://doi.org/10.1016/j.phytochem.2008.07.010
- FAIRBAIRN, J. W., & PICKENS, J. O. A. N. T. (1979). THE ORAL ACTIVITY OF Δ′-TETRAHYDROCANNABINOL AND ITS DEPENDENCE ON PROSTAGLANDIN E2. British Journal of Pharmacology, 67(3), 379–385. https://doi.org/10.1111/j.1476-5381.1979.tb08691.x
- Barrett, M. L., Gordon, D., & Evans, F. J. (1985). Isolation from cannabis sativa L. of cannflavin—a novel inhibitor of prostaglandin production. Biochemical Pharmacology, 34(11), 2019–2024. https://doi.org/10.1016/0006-2952(85)90325-9
- Werz, O., Seegers, J., Schaible, A. M., Weinigel, C., Barz, D., Koeberle, A., Allegrone, G., Pollastro, F., Zampieri, L., Grassi, G., & Appendino, G. (2014). Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition, 2(3), 53–60. https://doi.org/10.1016/j.phanu.2014.05.001
- Bautista, J. L., Yu, S., & Tian, L. (2021). Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega, 6(8), 5119–5123. https://doi.org/10.1021/acsomega.1c00318
- Salem, M. M., Capers, J., Rito, S., & Werbovetz, K. A. (2011). Antiparasitic Activity of C-Geranyl Flavonoids from Mimulus bigelovii. Phytotherapy Research, 25(8), 1246–1249. https://doi.org/10.1002/ptr.3404
- Huang, E. S., Strate, L. L., Ho, W. W., Lee, S. S., & Chan, A. T. (2011). Long-Term Use of Aspirin and the Risk of Gastrointestinal Bleeding. The American Journal of Medicine, 124(5), 426–433. https://doi.org/10.1016/j.amjmed.2010.12.022
- Erridge, S., Mangal, N., Salazar, O., Pacchetti, B., & Sodergren, M. H. (2020). Cannflavins – From plant to patient: A scoping review. Fitoterapia, 146, 104712. https://doi.org/10.1016/j.fitote.2020.104712
- Statista. (2020, November 5). Total U.S. cannabidiol (CBD) product sales 2014–2022. https://www.statista.com/statistics/760498/total-us-cbd-sales/
- Maccarrone, M. (2020). Phytocannabinoids and endocannabinoids: different in nature. Rendiconti Lince i. Scienze Fisiche e Naturali, 31(4), 931–938. https://doi.org/10.1007/s12210-020-00957-z
- Erickson, B. E. (2021, July 21). Cannabis industry gets crafty with terpenes. Chemical & Engineering News. https://cen.acs.org/biological-chemistry/natural-products/Cannabis-industry-crafty-terpenes/97/i29
- Price, G., & Patel, D. A. (2020, October 20). Drug Bioavailability – StatPearls – NCBI Bookshelf. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK557852/
- Ilyashov, A. (2019, January 30). Terpenes 411: What is Humulene? Medmen. https://www.medmen.com/blog/wellness/terpenes-411-what-is-humulene
- Trulieve. (2020, November 2). Everything you need to know about the terpene Humulene. https://www.trulieve.com/discover/blog/everything-you-need-to-know-about-the-terpene-humulene